*This post was updated in April 2020 to reflect the postponement of the EU MDR deadline.
In today’s fast changing regulatory landscape, it’s easy to get overwhelmed. Regulatory bodies are placing manufacturer data and submissions under the proverbial microscope to make sure nothing is amiss. Adopting a rigorous and transparent systematic literature review process is one way that regulatory professionals can ensure their data stands up to the scrutiny.
Systematic reviews use comprehensive, rigorous methods to identify, appraise, and synthesize all the available studies on a particular topic, they have traditionally been considered the gold standard for evidence-based medicine.
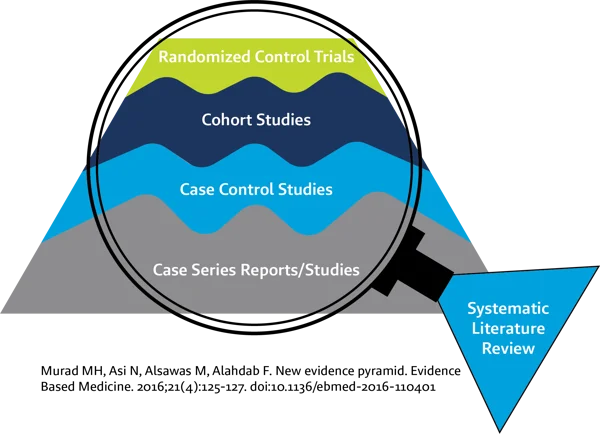
Today, systematic literature review (SLR) is increasingly mandated in other fields where the highest quality evidence is required to make important decisions about safety and efficacy. For example, regulatory authorities are now requiring a much more rigorous, repeatable, and transparent approach to the literature review component of medical device clinical evaluations.
Facing a much more involved literature review process, many device manufacturers are now asking: how do we produce the required evidence as efficiently as possible?
How does systematic literature review help?
Hands-down, the best way for regulatory professionals to meet the new expectations is by adopting a transparent, reproducible systematic literature review process and leveraging the latest technology to automate manual tasks (transparency and reproducibility are next to impossible with traditional literature review methods like spreadsheets).
By taking a systematic approach, device manufacturers can maximize their resources, improve efficiency, and cut time. It will facilitate training, consistency, and collaboration within your organization. Additionally, a systematic, transparent process will help minimize bias and maximize the reproducibility and utility of the review results to various stakeholders.
Systematic literature review essentials
So what are the essential steps to a methodologically sound SLR?
Step 1: The research question
Every subsequent part of your review will flow from your review questions, and well-defined review questions are an essential component to a systematic process.
When developing your research question, it’s important to make it as specific and clear as possible. To help define your question, use the PICO framework (Population, Interventions, Comparators and Outcomes).
This step may also involve scoping the literature to gauge the potential volume and type of data and balancing this with available resources and timelines. Scoping can help determine which sources (e.g. databases) are important for your needs.
Step 2: The search strategy
This step is also one of the most important parts of your SLR process because if it’s done poorly (e.g. an incomplete search) your review could be invalidated.
If you can, use a professional librarian/information specialist to help with this step. It’s also essential to document everything in this step, so your process remains transparent and reproducible. Guidelines such as PRISMA identify key points which should be recorded and reported.
Step 3: Screening
It’s a best practice to perform the screening process in duplicate to avoid errors.
To deal with the sheer volume of materials that need to be screened, most teams employ a multi-stage screening process to save both time and money. Automation is also a helpful tool that can be used to improve efficiency.
Expert Tip: Transparency in every step of your SLR is essential and reasons for exclusion should be tracked. A complete list of excluded studies is a common reporting requirement.
Step 4: Data extraction and appraisal
Data extraction is facilitated by the use of pre-developed, easy-to-use forms to collect the information you need. This makes it easier to train your team, can improve reviewer calibration, and help identify and resolve reviewer conflicts. Additionally, and importantly, it can result in cleaner data and improved efficiency.
Expert tip: Consider storing your references and their extracted data in a repository so you can leverage the work you have done.
Of course, not all evidence is created equal, and many types of systematic literature reviews require a systematic appraisal and weighting of the evidence. There are various accepted methods to appraise evidence, and this information should be collected with the same care as any other data: using systematic methods and by researchers with appropriate training.
Step 5: Synthesis
The type of synthesis you do depends on your data and overall goal. For example, not all SLRs end with a meta-analysis. Researchers need to consider the heterogeneity of their research before determining the best way to synthesize their data.
Step 6: Reporting
Reporting is the final step in your systematic literature review process and requirements may differ. For medical device clinical evaluation reports, for example, notified bodies will look for a transparent description of your methods of screening, data extraction, and synthesis. They will also look for summaries of the study data where conclusions were drawn, and more. Guidelines such as the PRISMA Statement can be useful here too.
The role of SLR software
Traditional SLR tools such as spreadsheets are not enough to help regulatory professionals meet new standards. Today’s researcher needs a systematic process that is transparent and reproducible in order to produce the required data to support regulatory decisions.
This is where systematic literature review software comes in – here are just a few reasons why it is essential for any SLR toolkit. It can:
- Automate manual processes to save time and reduce errors.
- Help enforce a prescriptive process for your review, which is helpful for training, cleaner data, and opportunities for data-reuse in future reviews and updates.
- Facilitate collaboration with features for real-time team and project management.
Provide an audit trail with features such as tracking, version control, time stamping, which is essential for regulatory-compliance. - Allow you to store data and reuse it, which is something that will save significant time and effort in future reviews and updates.
For regulatory professionals, SLR software is by far the best way to ensure that clinical evidence meets all the standards required by notified bodies.
By following the steps outlined here, you’ll be well on your way to better evidence!








