About Systematic Reviews
PRISMA Guidelines for
Systematic Review


Automate every stage of your literature review to produce evidence-based research faster and more accurately.
Poor reporting and transparency have plagued systematic reviews for decades. To address these ongoing issues, experienced authors and methodologists came together to create a solution: PRISMA.
What Is PRISMA?
PRISMA – Preferred Reporting Items for Systematic reviews and Meta-Analyses – is an evidence-based minimum set of items for reporting. PRISMA is an international initiative created to address the lack of transparent, well-documented review methodologies reported in review papers.
The primary focus of PRISMA is the reporting of reviews evaluating the effects of interventions, but it can also be used to report systematic reviews with other objectives (eg, evaluating diagnosis, etiology, prevalence, or prognosis).
The PRISMA 2020 guidelines are relevant for mixed-methods systematic reviews (including quantitative and qualitative studies). However, reporting guidelines addressing the presentation and synthesis of qualitative data should also be consulted.
PRISMA Guidelines
In the 1980s, research by Cynthia Mulrow uncovered deficiencies in the quality of systematic review reports. She explained that the lack of quality assessment of primary studies was a major pitfall in these reviews. Her work started a drive to improve the standards of reporting, which led to the publication of the PRISMA statement.
In 2009, the first PRISMA statement was published as a standard operating procedure that covered a minimum set of items that must be presented in the review. This minimum set of items are known today as the PRISMA guidelines.
In 2020, the PRISMA guidelines were updated to reflect the changing reality of the current research climate, but the 2020 PRISMA statement retains the core of the PRISMA guidelines. So, what makes up the PRISMA guidelines systematic review?
The PRISMA Checklist
The PRISMA checklist is a 27-item list written by experienced authors and methodologists to help improve the transparency of systematic review reporting. The checklist covers every section of a systematic review and provides an explanation of the meaning and rationale for each item. This gives authors a clearer understanding of why each item is important for the systematic review.
The checklist gives reviewers and authors the 27 fundamental items that must be reported in their reviews. Each item on the list can be checked off as they are completed by reviewers; the page number of the review can also be added for ease of reference.
The PRISMA checklist guides reviewers and authors through the small but necessary details that make up a good systematic review. Authors and researchers are encouraged to check the guideline early in the writing process to ensure that all necessary information will be present.
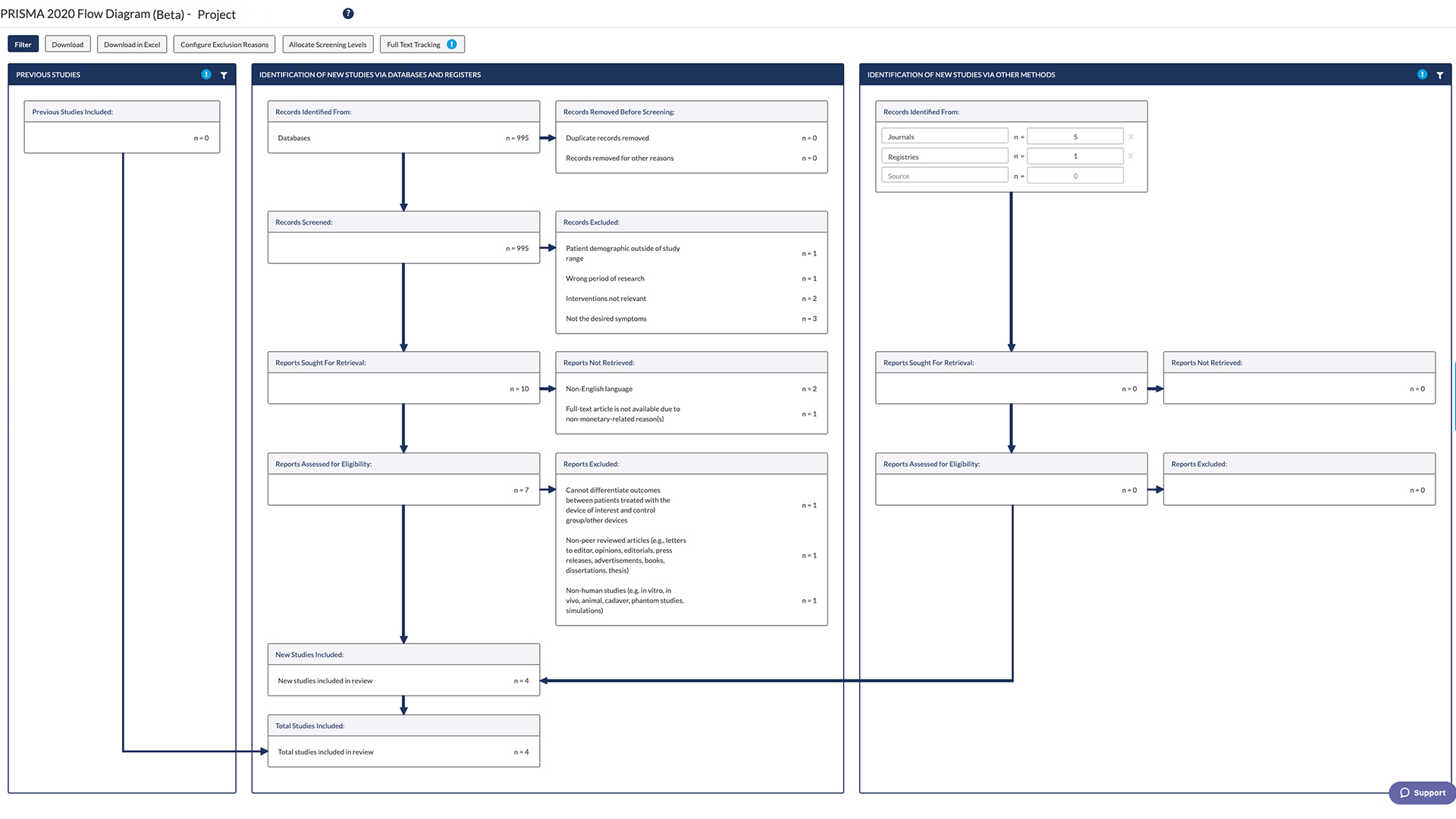
The PRISMA Flow Diagram
The PRISMA flow diagram is item 16A on the checklist; it tells reviewers to “Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram.”
The PRISMA flow diagram or flowchart is a visual report of the decisions that the review team took as they assessed citations for possible inclusion in the review. It simplifies the reporting of study selection to its simplest components and graphically communicates the necessary information quickly and effectively.
The flow diagram is broken down into 4 separate phases, including identifying the articles for review, screening the articles for review, deciding on the studies’ eligibility, and finalizing the list of studies to include in the systematic review. PRISMA has provided a template for the flow diagram, but you can learn more in our article on how to do a PRISMA flow diagram.
Learn More About DistillerSR
(Article continues below)
The PRISMA Extensions
The PRISMA extensions are guidelines or checklists provided by PRISMA to supplement the main checklist, and are specific to a certain aspect, section, or type of review. The extensions are used for abstracts, diagnostic test accuracy studies, health equity reviews, literature searches, meta-analyses of individual patient data, network meta-analyses, protocols, reviews of harm outcomes, and scoping reviews.
Purpose of PRISMA Guidelines
The PRISMA guideline is an important tool that helps both new and established authors avoid the pitfalls that can undermine systematic reviews. The guideline ensures that reviewers and authors achieve clarity and transparency in every part of their systematic review. In addition, following the PRISMA guideline allows readers to assess the strengths and weaknesses of a study, and facilitates the replication of the review methods.